Effective Ways to Find Limiting Reactant for Accurate Chemical Reactions in 2025
In the world of **chemical reactions**, one concept that stands out is the **limiting reactant**—an essential component that can significantly impact the outcome of a reaction. Understanding how to determine the limiting reactant is vital for achieving maximum efficiency and accuracy in **chemical yield**. This article explores effective techniques and considerations for identifying limiting reagents while incorporating the principles of **stoichiometry**.
The Importance of Identifying Limiting Reagents
Identifying **limiting reagents** is crucial in optimizing chemical reactions, as it helps in understanding the maximum quantity of product that can be formed. When reactants are combined, they react in specific **reactant ratios** as dictated by balanced **chemical equations**. The reactant that is entirely consumed during the reaction dictates the extent of the reaction and thus plays a pivotal role in **reaction completion**. Without recognizing the limiting reactant, one may overestimate the **overall reaction yield**, leading to disappointments in results and wasted resources.
Understanding Stoichiometry in Chemical Reactions
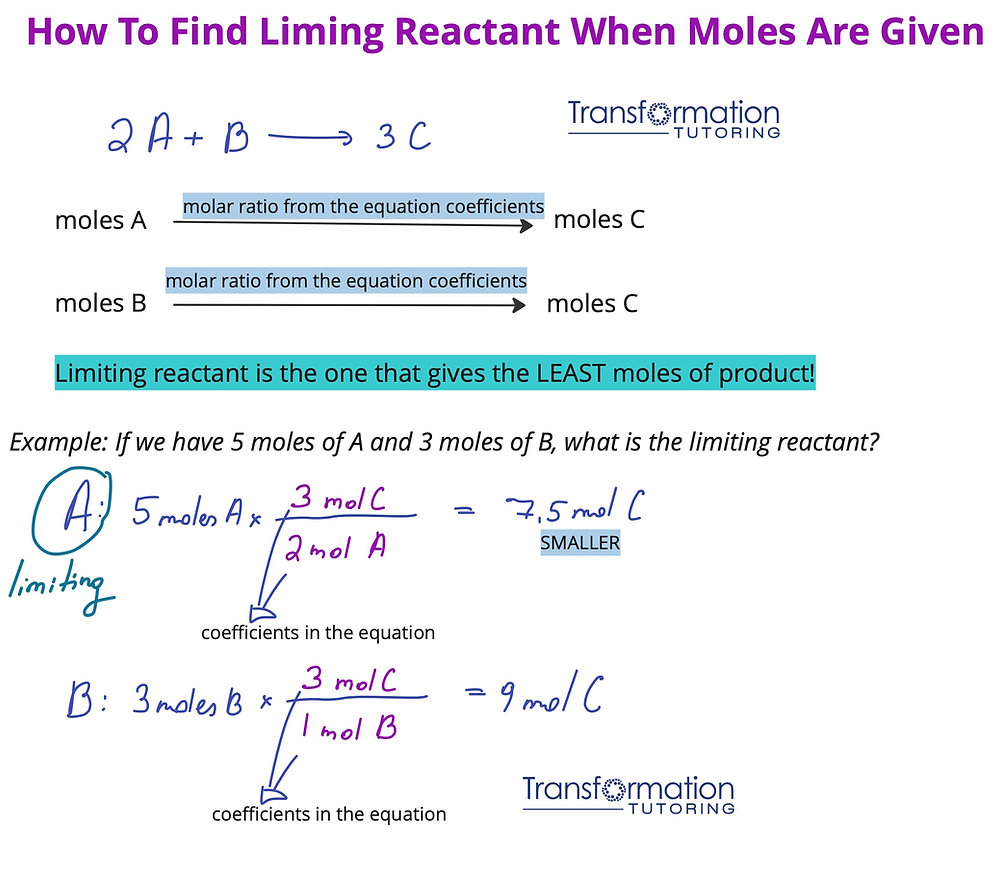
**Stoichiometry** is the foundation of understanding the relationships between **reactants and products** in a chemical reaction. By analyzing **moles of reactants** and using **balanced chemical equations**, one can efficiently assess the volume or mass of reactants required. For example, consider the reaction between hydrogen (H₂) and oxygen (O₂) to form water (H₂O). The balanced equation is:
2H₂ + O₂ → 2H₂O
This equation indicates that two moles of hydrogen react with one mole of oxygen to produce two moles of water. If you have three moles of H₂ and one mole of O₂, the limiting reactant is O₂ since you would need four moles of H₂ to fully react with the available O₂. This clear demonstration shows the practical usage of stoichiometry in determining limiting factors.
Analysis of Reactant Relationships
Analyzing the relationships between the different **reactants** involved in a reaction is key to determining the limiting reactant. **Chemical calculations** utilizing **reactant concentrations** allow chemists to evaluate how much of each reactant is available compared to what is needed according to the stoichiometric ratios. For instance, if your reaction requires an **excess reactant**, determining the limiting reactant lets you strategically calculate the amount of reactant to stoichiometrically use, ensuring maximum yield. Additionally, careful consideration should be given to **molar ratios** and **empirical formulas** to validate the accurate mixing of reactants.
Practical Techniques for Determining the Limiting Reactant
Practical techniques are essential for determining which reactant is limiting. One practical approach is through **calculation methods** that simplify the analysis of reactants in various forms, whether solid, liquid, or gas.
Mole Conversion Techniques
**Mole conversions** are among the most effective techniques for identifying limiting reactants. By converting the mass of each reactant into moles using their molar mass, you can evaluate how many moles of product each reactant can produce. The steps generally involve:
- Calculating moles of each reactant based on mass and molar mass.
- Applying the **stoichiometric coefficients** from the balanced equation.
- Comparing the calculated mole ratios to identify which reactant runs out first.
This approach ensures precision while analyzing **reaction efficiency**, allowing chemists to derive clear conclusions from their experimental data.
Using Chemical Equations for Efficient Calculations
Utilizing **chemical equations** enables the systematic breakdown of reactants into their necessary components, revealing their relationships and any limitations present in the reaction dynamics. By adhering to a set procedure—such as balancing your equations first and then applying methods such as the **reaction stoichiometry**—you can avoid errors and streamline your experimental setup.
Challenges and Considerations in Limiting Reactant Analysis
Yet, there might be challenges in determining the limiting reactant during reactions. Factors such as the purity of reactants, mixed solutions, and environmental conditions can adversely affect the efficiency of the reaction.
Impacts of Reactant Concentrations
The concentration of each reactant can significantly alter its effectiveness in reactions. Often, chemists find themselves tackling complex mixtures where concentrations vary widely. It’s crucial to measure the **solution concentrations** or utilize **concentration tables** to accurately determine how much can feasibly react. Poorly mixed reactants can lead to non-ideal outcomes, emphasizing the necessity of careful laboratory procedures.
Dealing with Experimental Limitations
**Laboratory experiments** inherently come with limitations due to equipment, environmental factors, or even human error. Understanding how these limitations affect the relationship between reactants can help in overcoming challenges and yielding more reliable results. Monitoring factors such as pressure and temperature might also offer insights into how optimally a reaction proceeds, as they directly correlate with reaction efficiency.
Key Takeaways
- Identifying the **limiting reactant** is essential for predicting the outcome of chemical reactions accurately.
- Utilizing mole conversions and analysis of **reactant concentrations** facilitates efficient **reactant calculations**.
- Balancing **chemical equations** prior to computations ensures clear understanding and minimization of errors.
- Always consider the **empirical formulas** and conditions affecting your experiment to derive valid conclusions.
- Recognizing **limiting factors in reactions** allows chemists to optimize their methodologies for better experimental results.
FAQ
1. What is a limiting reactant and why is it important?
A limiting reactant is the substance that is entirely consumed when a chemical reaction goes to completion. It is pivotal to determining how much product can be produced, thereby ensuring precise calculations in any quantitative analysis in chemistry.
2. How do you find the limiting reagent using moles?
To find the limiting reagent using moles, first convert the mass of each reactant into moles. Then, compare the mole ratio of available reactants to the ratio required in the balanced equation to see which reactant is in excess and which gets consumed first.
3. What factors affect the identification of the limiting reactant?
Factors such as the purity of reactants, concentration, and environmental conditions like temperature and pressure can affect how reactants behave in a reaction, influencing the identification of the limiting reactant.
4. Can you provide an example of limiting reactant in a practical chemistry scenario?
In a reaction involving sodium chloride and silver nitrate to form silver chloride, if we start with 2 moles of sodium chloride and 1 mole of silver nitrate, upon analyzing the balanced equation, silver nitrate would be the limiting reagent since the reaction stoichiometry requires equal moles to fully react.
5. How do stoichiometric calculations assist in finding limiting factors in reactions?
**Stoichiometric calculations** allow chemists to analyze the exact proportions required for reactions, helping them understand which reactant will run out first based on the quantities available. This offers a precise way to optimize yields and reactant efficiency in experiments.
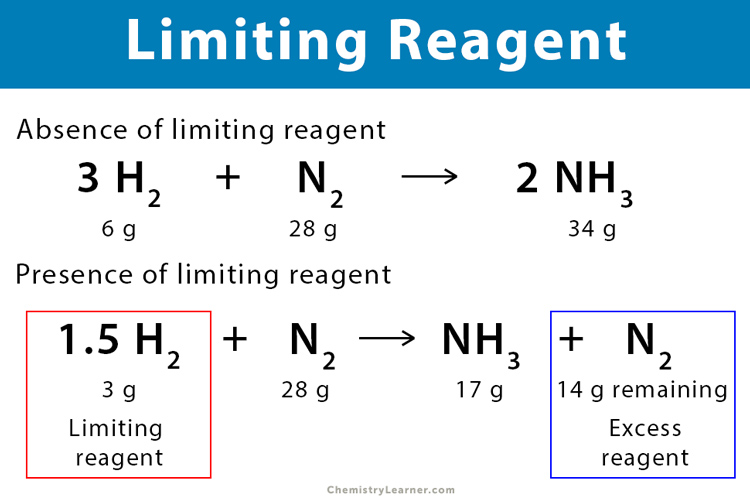
6. What is the effect of excess reactants on the outcome of a reaction?
Excess reactants are those that remain after the limiting reactant has been consumed. They do not affect the maximum yield of the product but can result in wasted materials. Understanding excess reactants can help better plan for experiments by minimizing waste.
7. What resources can I use for further learning about limiting reactants?
Educational resources such as textbooks on **analytical chemistry**, online tutorials, and laboratory manuals provide rich information for better understanding **limiting reactants**, **stoichiometric principles**, and practical applications in real-world scenarios.